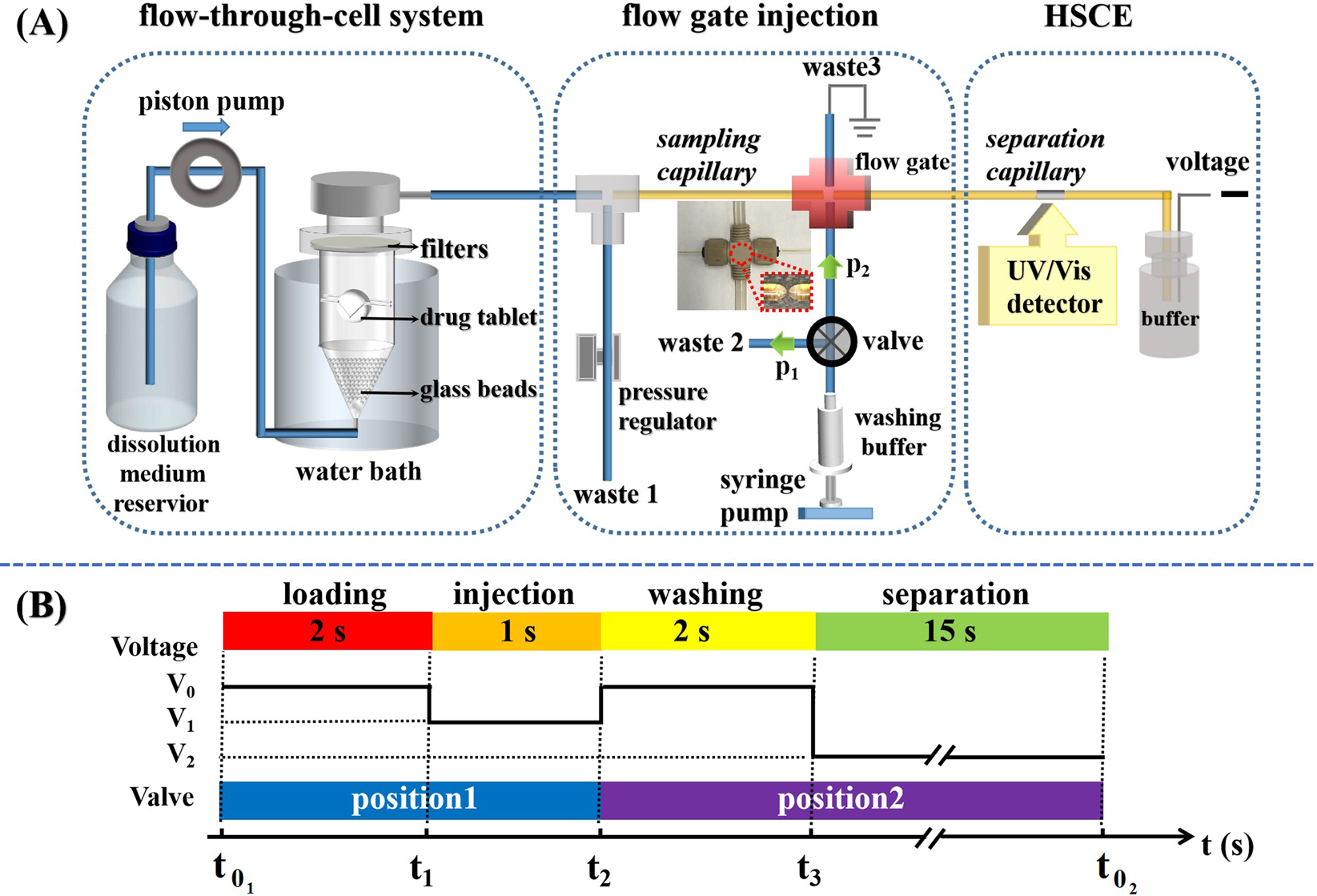
Automatic Dissolution Testing with High-Temporal Resolution for Both Immediate-Release and Fixed-Combination Drug Tablets | Scientific Reports

The influence of different mechanical stress on the release properties of HPMC matrix tablets in sucrose-NaCl media - ScienceDirect

An In Vitro Dissolution Method for Testing Extended-Release Tablets Under Mechanical Compression and Sample Friction - Journal of Pharmaceutical Sciences

PDF) A sensitive dissolution test method for the development and validation of levetiracetam tablets by reverse phase-HPLC technique | C.M. KARRUNAKARAN - Academia.edu
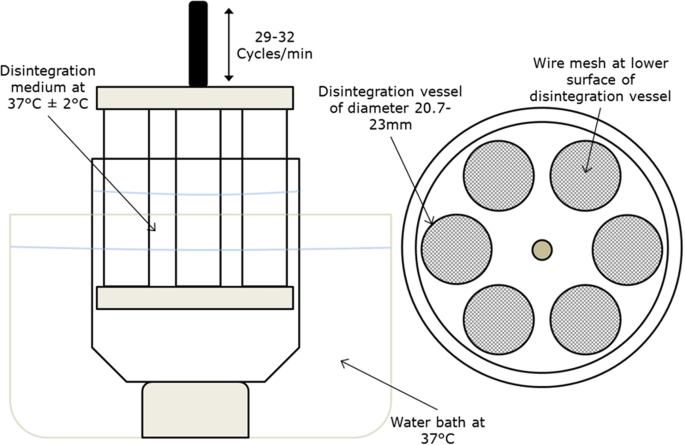
Conceptualisation, Development, Fabrication and In Vivo Validation of a Novel Disintegration Tester for Orally Disintegrating Tablets | Scientific Reports

PDF) DEVELOPMENT AND VALIDATION OF DISSOLUTION TEST METHOD FOR ALISKIREN IN TABLET BY HPLC | parag khairnar - Academia.edu

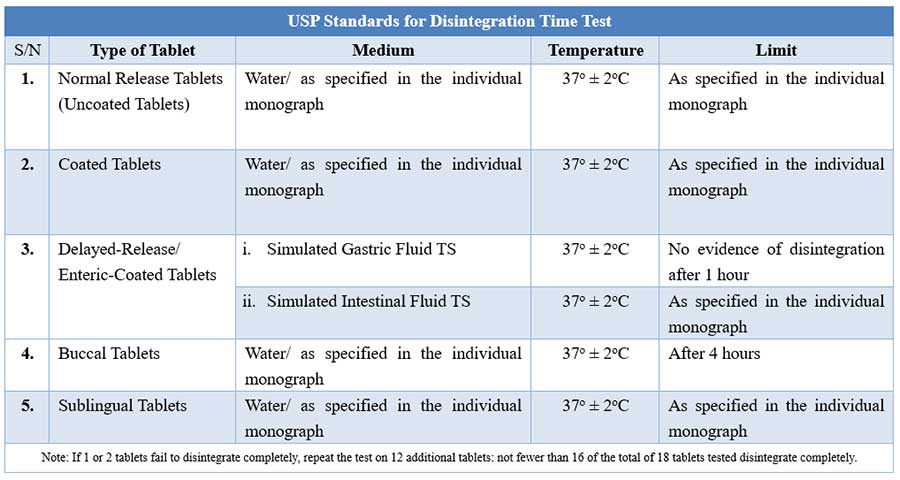

![PDF] History and Evolution of the Dissolution Test | Semantic Scholar PDF] History and Evolution of the Dissolution Test | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e445cfd8d997cbb7a15cc00e6cf58d6cb6415222/5-Figure2-1.png)





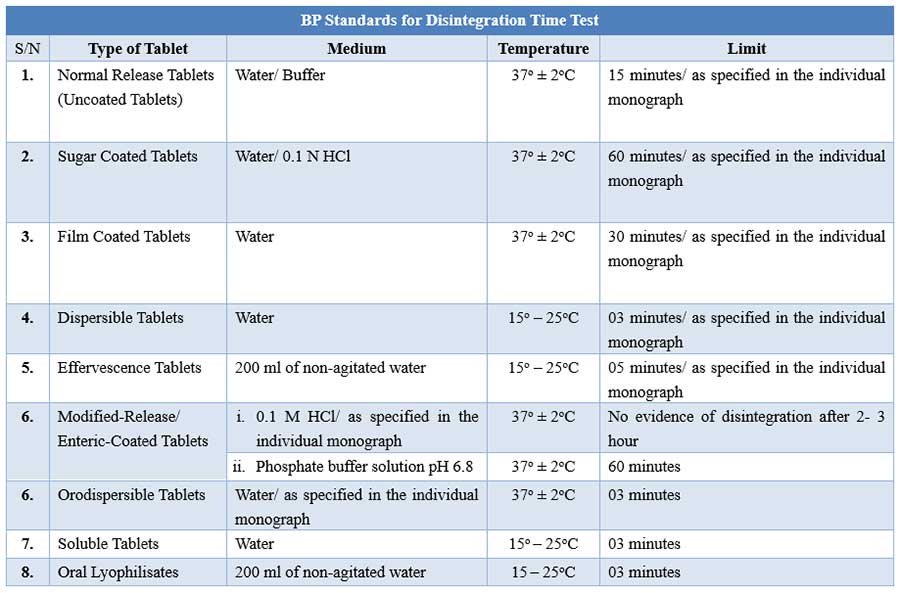
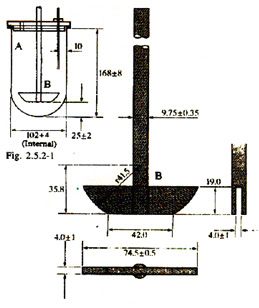

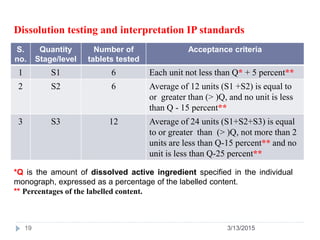


![PDF] The Significance of disintegration testing in pharmaceutical development | Semantic Scholar PDF] The Significance of disintegration testing in pharmaceutical development | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/92a193f174837cd54c0ca8eb588ad0f3e936abc1/5-Table3-1.png)
